
Bacteria can survive under varied conditions (called Bacterial Stress Response), and to overcome adverse situations, the changes must be sensed by bacteria and mount reactions in protein activity and gene expression.
The stress reaction in bacteria involves a system of components that behave against the stimulation.

Compounds can respond simultaneously to many different stresses, and the variety of stress response systems in bacteria interact (cross-talk) with every other.
- What is Mendelian Law and Mendelism?
- Introduction to genetics and heredity
- Mutations: What is Mutations and its types
- Down Syndrome: Genetic Disorder of Humans
A network of systems that are international contributes to an efficient and coordinated reaction.
These systems regulate the manifestation of effectors that maintain the equilibrium of the balance under the ailments.
Bacteria can survive under diverse environmental conditions, and to overcome these adverse and changing conditions, bacteria must sense the changes and mount appropriate responses in gene expression and protein activity.
The stress response in bacteria involves a complex network of elements that act against the external stimulus. Bacteria can react simultaneously to a variety of stresses, and the various stress-responsive systems interact (cross-talk) with each other.
A complex network of global regulatory systems leads to a coordinated and efficient response.
These regulatory systems govern the expression of more effectors that maintain the stability of the cellular equilibrium under the various conditions.
- What are the Basic Conditions and Types of Bacterial Growth?
- What is Bacterial Stress Response?
- Microbial Physiology: Yields of Microbes
Stress-responsive systems can play an essential role in the virulence of pathogenic organisms.
In bacteria some of the most important stress-responsive systems are:
- Heat shock response, controlled by the sigma factor – (sigma 32)
- Envelope stress response, controlled mainly by the sigma factor (sigma-E) and the Cpx two-component system
- Cold shock response, which governs the expression of RNA chaperones and ribosomal factors;
- General stress response, which depends on the sigma factor – (sigma-S)
- The (P)ppGpp-dependent stringent response which reduces the cellular protein synthesis capacity and controls further global responses upon nutritional downshift
Bacterial Stress Response
Further examples include the secretion of a protein domain, TauD, to breakdown taurine into sulfur in times of need and YodA in toxic metal response.
1. Tau-D protein domain
In molecular biology, TauD refers to a protein domain that in many enteric bacteria is used to breakdown taurine (2-aminoethane sulphonic acid) as a source of sulfur under stress conditions.
In essence, they are domains found in enzymes that provide bacteria with an important nutrient.
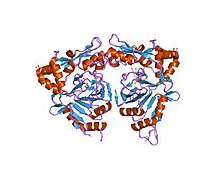
Function:
- This protein family consists of TauD/TfdA taurine catabolism dioxygenases. The Escherichia coli tau.D gene is required for the utilization of taurine (2-aminoethane sulphonic acid) as a sulfur source and is expressed only under conditions of sulfate starvation.
- TauD is an alpha-ketoglutarate-dependent dioxygenase catalyzing the oxygenolytic release of sulfite from taurine.
- The 2,4-dichlorophenoxyacetic acid/alpha-ketoglutarate dioxygenase from Burkholderia sp. (strain RASC) also belongs to this family. TfdA from Ralstonia eutropha (Alcaligenes eutrophus) is a 2,4-D monooxygenase.
Structure
This structure has some alpha helices and beta sheets.
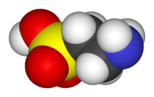
2. Taurine
Taurine, or 2-aminoethanesulfonic acid, is an organic acid widely distributed in animal tissues. It is a major constituent of bile and can be found in the large intestine and accounts for approximately 0.1% of total human body weight. Taurine has many fundamental biological roles such as conjugation of bile acids, antioxidation, osmoregulation, membrane stabilization and modulation of calcium signaling.
It is essential for cardiovascular function, and the development and function of skeletal muscle, the retina, and the central nervous system.
Taurine is unusual among biological molecules in being a sulfonic acid, while the vast majority of biologically occurring acids contain the more weakly acidic carboxyl group. While taurine is sometimes called an amino acid, and indeed is an acid containing an amino group.
It is not an amino acid in the usual biochemical meaning of the term, which refers to compounds containing both an amino and a carboxyl group.

Sources of Taurine
1. Dietary intake
Taurine occurs naturally in food, especially in seafood and meat. The mean daily intake from omnivore diets was determined to be around 58 mg (range from 9 to 372 mg) and to be low or negligible from a strict vegan diet. In another study, taurine intake was estimated to be less than 200 mg/day, even in individuals eating a high-meat diet. According to another study, taurine consumption was estimated to vary between 40 and 400 mg/day.
2. Biosynthesis
Taurine is a major constituent of bile and can be found in the large intestine and the tissues of many animals, including humans.
Mammalian taurine synthesis occurs in the pancreas via the cysteine sulfinic acid pathway. In this pathway, the sulfhydryl group of cysteine is first oxidized to cysteine sulfinic acid by the enzyme cysteine dioxygenase.
Cysteine sulfinic acid, in turn, is decarboxylated by sulfinoalanine decarboxylase to form hypotaurine.
It is unclear whether hypotaurine is then spontaneously or enzymatically oxidized to yield taurine.
3. Nutritional significance
A study of mice hereditarily unable to transport taurine suggests that it is needed for proper maintenance and functioning of skeletal muscles.
Also, it has been shown to be effective in removing fatty liver deposits in rats, preventing liver disease, and reducing cirrhosis in tested animals.
There is also evidence that taurine is beneficial for adult human blood pressure and possibly, the alleviation of other cardiovascular ailments (in humans suffering essential hypertension, taurine supplementation resulted in measurable decreases in blood pressure.
- What is Mendelian Law and Mendelism?
- Introduction to genetics and heredity
- Mutations: What is Mutations and its types
- Down Syndrome: Genetic Disorder of Humans
Taurine is regularly used as an ingredient in energy drinks, with many containing 1000 mg per serving, and some as much as 2000 mg. A 2003 study by the European Food Safety Authority found no adverse effects for up to 1,000 mg of taurine per kilogram of body weight per day.
A review published in 2008 found no documented reports of negative or positive health effects associated with the amount of taurine used in energy drinks, concluding that “The amounts of guarana, taurine, and ginseng found in popular energy drinks are far below the amounts expected to deliver either therapeutic benefits or adverse events.”
- Synthetic taurine is obtained from isethionic acid (2-hydroxyethanesulfonic acid), which in turn is received from the reaction of ethylene oxide with aqueous sodium bisulfite.
- Another approach is the reaction of aziridine with sulfurous acid.
- It leads directly to taurine.
- In 1993, approximately 5,000–6,000 tons of taurine were produced for commercial purposes; 50% for pet food manufacture, 50% in pharmaceutical applications.
- As of 2010, China alone has more than 40 manufacturers of taurine.
- Most of these enterprises employ the ethanolamine method to produce a total annual production of about 3,000 tons.
4. Toxicity
- Taurine is involved in some crucial physiological processes.
- However, the role of taurine in these processes is not understood, and the influence of high taurine doses on these processes is uncertain.
- A substantial increase in the plasma concentration of growth hormone was reported in some epileptic patients during taurine tolerance testing (oral dose of 50 mg/kg BW/day), suggesting a potential to stimulate the hypothalamus and to modify neuroendocrine function.
- There is an indication that taurine (2 g/day) has some function in the maintenance and possibly in the induction of psoriasis.
- It may also be necessary to take into consideration that the absorption of taurine from beverages may be more rapid than from foods.
3. Zin-T protein domain (or) YodA
In molecular biology, ZinT protein domain which was formerly known as YodA refers to a member of a family of prokaryotic domains. This protein family was first thought to be part of the bacterial response to toxic heavy metal cadmium by binding to the metal to ensure its elimination. However, more recent studies have opposed this hypothesis. This protein domain is found exclusively in bacteria.
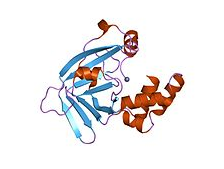
Function
- The precise role of this protein domain remains unknown.
- It was initially found in the cadmium.
- Further studies have found that it binds to cadmium, zinc, nickel, and mercury, but not other common metals such as cobalt, copper, iron, and manganese.
- It has been thought to be part of the bacterial stress response since Zin-T expression has been found to be induced by cadmium, and more recently, hydrogen peroxide and then is localized to the cytoplasm.
Structure YodA
This protein domain has a central structural feature of an antiparallel up-down beta-barrel with some smaller alpha-helices.
- What are the Clinical significance of Purine metabolism?
- What are the Secondary Structure of Proteins?
- ATP cycle: Structure and role of ATP in biochemical reactions
Share this Bacterial Stress Response topic with your friends on social media who are preparing for higher examinations. Sharing is Caring.
