
Thermodynamics is the branch of physical chemistry that deals with energy changes and Biochemical thermodynamics (or biochemical energetics of bioenergetics as it is also called) is the field of biochemistry concerned with the energy transformation and use of energy by living cells (Energy transformation). The chemical reactions occurring in living beings (or biochemical reactions) are associated with the liberation of lower energy levels (law of conservation of energy).
It is the quantitative study of energy transformation, energy relationships, and energy conversions in biological systems.
All organisms need free energy to keep themselves alive and functioning. The source of energy is just one; solar energy. Only plants use that energy directly. What the organisms use is chemical energy in the form of foods.

The very first conversion of solar energy into chemical energy is the sugar molecule. On one side the conversion of solar energy into chemical energy with the help of photosynthesis happens, and on the other hand, this photosynthesis makes it possible with the passage of time on earth to accumulate free oxygen in the earth’s atmosphere making possible the evolution of respiration.
Respiration is important for bioenergetics as it stores the energy to form a molecule ATP (Adenosine triphosphate). This molecule is a link between catabolism and anabolism. The process of photosynthesis is helpful in understanding the principles of energy conversion i.e. bioenergetics (Energy Transformation).
Photosynthetic organisms and plants capture solar energy and synthesize organic compounds. It is a way of energy input. Energy stored in these organic compounds that are mainly sugars can be used later as a source of energy.
Photosynthesis after respiration provides glycolysis, a major substrate, and later this glycolysis with further respiration provides energy in very controlled processes. So respiration and photosynthesis are the main processes dealing with bioenergetics.
What is an Energy Transformation?
Energy can be converted from one form to another. Chemical energy can be tapped when chemical reactions rearrange the atoms of molecules in such a way that potential energy stored in the molecules is converted to kinetic energy. This transformation occurs.
Definition of energy transformatio is
Energy transformation, also known as energy conversion, is the process of changing energy from one form to another. In physics, energy is a quantity that provides the capacity to perform work (e.g. Lifting an object) or provides heat. In addition to being converted, according to the law of conservation of energy, energy is transferable to a different location or object, but it cannot be created or destroyed.
For example. in the engine of an automobile, when the hydrocarbons of gasoline react explosively with oxygen, releasing the energy that pushes the ‘Piston’. This is the live example of energy transformation.
Energy can never be created or destroyed – it can only change from one form to another.
Similarly, chemical energy fuels organisms. cellular respiration and another other pathway unleash energy stored in sugar and other complex molecules and make that energy available for cellular work. The chemical energy stored in the fuel molecules had itself been converted from light energy by plans during “Photosynthesis”.
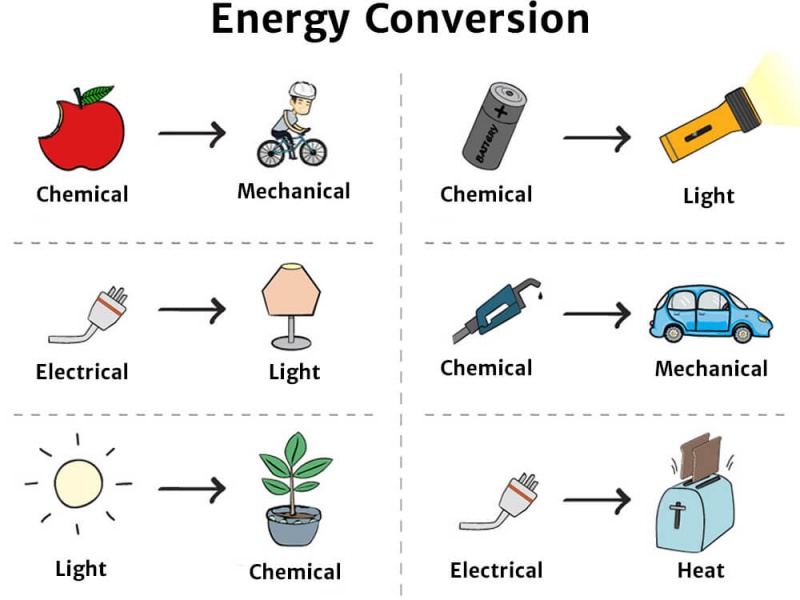
Energy Transformation in the Living system
For chemical reactions occurring in solution, we can define a system as all of the reactants and products present, the solvent, and the immediate atmosphere, in short, everything within a defined region of space.
The system and its surroundings together constitute the Universe. The system has been classified into three levels based on energy transformation. Let us see the energy transformation in nature.
- Closed System
- Isolated System
- Open System
1. Closed System
If the system exchanges neither matter nor energy with its surroundings, it is said to be a “Closed system”. E.g: Boiling water in a cold beaker, Chlorophyll system. In the system, no change in matter, but can exchange heat through the beaker edges.
Δm = 0
ΔQ ≠ 0
Δm = Change in MASS
ΔQ = Change in HEAT
2. Isolated System
If the system exchanges energy but not matter with its surrounding. it is an “Isolated system”. Eg: Earth System.
3. Open System
If it exchanges both energy and material with its surroundings, it is an “Open system”. Eg: Boiling water in open beaker in the laboratory, Cell system. In the system, both energy and material can exchange with its surroundings.
Δm ≠ 0
ΔQ ≠ 0
Living organisms is an open system. it exchanges both matter and energy with its surrounding. Living organisms use either of two strategies to derive energy from their surroundings.
- They take up chemical fuels from the environment and extract energy by oxidizing them.
- They absorb energy from sunlight.
Focus points on Energy transformation
- Living cells are “chemical engines” that function at constant temperatures.
- Living cells at any given moment exist in a steady state in which the rate of input of matter equals the rate of output of matter.
- Organisms transfer energy and matter from their surroundings.
- Organisms are never at equilibrium with their surroundings.
- Organisms are islands of low entropy in an increasingly random universe.
Understanding Terms: Before studying thermodynamics, we want to understand some terms.
- System: The Collection of matter under study and refer to the rest of the universe.
- Surrounding: Everything outside of the system.
- Energy: Energy is the capacity to do work
- Entropy: The randomness of the components of a chemical system is expressed as “Entropy”, denoted simply as “S”.
- Enthalpy: The heat content of a system, denoted simply as “H”.
What is energy conservation?
There are many different types of energy, including kinetic, potential, gravitational and electrical. Energy can be transferred (move from one location to another) and it can change (transform) from one type to another – but the total amount of energy is always conserved, i.e. it stays the same.
Free Energy Concept
Energy is defined as the capacity to do work, which is the product of a given force acting through a given distance.
Work = Force X Distance
In living organisms, the following are some of the sources of energy and forms of biological work. Here are the basic examples of energy transformation.
- Muscle Contraction
- Synthesis of Biomolecules
- Membrane function
- Generation and conduction of nerve impulse
Every biological work requires energy. The stored chemical energy is utilized to perform biological work. The input in a process is called the Energy and the output is Work. Chemical energy stored in a compound can be utilized to do the form of work, such as electrical, mechanical or heat. Energy and work can be measured quantitatively. The most common unit in biology is Calorie.
Calorie: The amount of flow energy required raising the temperature of one gram of water by degree centigrade. The other units are “erg” and “joule”. (One calorie =4.8 joules and One Joule = 107 ergs).
Bioenergetics is the quantitative study of energy transformations that occur within the living cells. This follows the laws of thermodynamics.
